
The following blog post is for entertainment and informational purposes only. It is not intended to provide medical advice or diagnosis. Please consult your doctor before making any health-related decisions.
Different pharmacological properties and possible therapeutic applications of Rapamycin have captivated researchers for decades. The biological activity of this molecule is provided by intricate Rapamycin chemical structure.
In this post, we begin a journey to unravel the structure of Rapamycin. We will describe its unique features and the implications for drug development and medical research.
Macrocyclic Rapamycin Structure
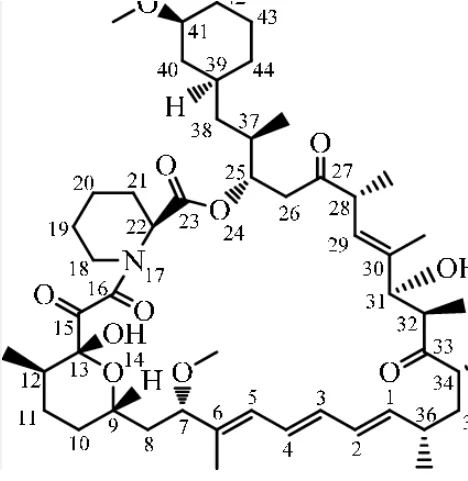
The macrocyclic composition lies at the heart of the Rapamycin chemical structure and biological activity. This molecule contains a large cyclic ester ring. This ring is formed through the intricate assembly of precursor molecules in a biosynthetic pathway. It is known as polyketide synthesis.
Major Features of the Macrocyclic Structure of RapamycinSize and Rigidity
The macrocyclic ring of this molecule is exceptionally large. It comprises 31 atoms. This size imparts significant rigidity to the molecule. So, it fosters its stability and conformational constraints.
The rigid structure of Rapamycin maintains the bioactivity of this molecule and facilitates its interactions with target proteins.
Ester Linkages
A series of ester linkages between adjacent carbon atoms form the macrocyclic ring. These ester bonds help to stabilize the macrocycle and maintain its structural integrity.
Ester linkages can also be cleaved by enzymatic processes. The absorption and elimination of these molecules from the organism are provided.
Hydroxyl Groups
Along the macrocyclic ring, the Rapamycin structure contains several hydroxyl (-OH) groups. They are attached to carbon atoms. These groups foster the general polarity and solubility of the molecule. They also provide interactions with other molecules.
Hydroxyl groups can also participate in hydrogen bonding and other non-covalent interactions with biological targets.
Chirality
This single stereoisomer has a defined configuration of chiral centers within the macrocyclic Rapamycin structure.
The precise Rapamycin chemical structure defines its biological activity and interactions with target proteins. Even small changes in stereochemistry can significantly impact the pharmacological properties of this drug.
Functional Groups
The macrocyclic Rapamycin structure contains other functional groups. There are carbonyl groups and alkene moieties. These functional groups foster the general chemical reactivity and biological activity of this molecule. They allow it to interact with specific targets and exert its pharmacological impacts.
The foundation for its biological activity and pharmacological properties is the macrocyclic Rapamycin structure. By acknowledging the intricate arrangement of atoms, researchers can gain insights into the mechanisms of operation of this molecule.
They can explore its possible applications in drugs and biotechnology.
Polyketide Synthesis
Polyketide synthesis is the intricate biochemical pathway responsible for the assembly of the complex macrocyclic structure of Rapamycin. This process involves the stepwise condensation of simple precursor molecules by a multifunctional enzyme complex. This is polyketide synthase (PKS).
Now we will introduce an overview of the PKS of the Rapamycin structure.
Initiation
PKS begins with the initiation of the PKS enzyme complex. This process involves the loading of a starter molecule onto an acyl carrier protein (ACP) domain within the PKS.
Chain Elongation
The starter molecule is loaded onto the ACP domain. Then successive acetate and propionate units are added to the growing polyketide chain through a series of condensation reactions. Each condensation reaction is catalyzed by specific enzyme domains within the PKS of the structure of Rapamycin.
Changes and Cyclization
The polyketide undergoes different changes and cyclization reactions to introduce structural diversity and complexity. These changes include oxidation, lowering, dehydration, and cyclization reactions catalyzed by specialized enzyme domains within the PKS of the structure of Rapamycin.
Macrocyclization
The final step in the PRS involves the macrocyclization of the polyketide chain to form the large cyclic ester ring characteristic of the Rapamycin structure. This process is accelerated by a specific enzyme domain within the PKS.
The macrocyclization process results in the formation of the 31-membered ring structure. It defines the molecule’s macrocyclic lactone backbone.
Release and Processing
After the macrocyclization reaction, the mature molecule of this molecule is liberated from the PKS enzyme complex. Additional processing steps can occur to yield the final bioactive form of Rapamycin structure.
Overall, the PKS of the Rapamycin structure is highly orchestrated. The complex process involves the coordinated action of different enzyme domains within the PKS enzyme complex.
Through a series of condensation, changes, and cyclization reactions, simple precursor molecules are transformed into the intricate macrocyclic structure of Rapamycin. They confer upon it its unique chemical and pharmacological properties.
Functional Groups

This molecule contains several functional groups. They foster its biological activity and pharmacological impacts.
Macrolactone Ring
The large cyclic ester ring confers stability and conformational rigidity to the molecule.
Discrete Hydroxyl Groups
The molecule contains several hydroxyl (-OH) groups at different positions along the Rapamycin structure. They promote its interactions with biological targets and the possibility of chemical changes.
Ester Groups
Ester linkages are present within the macrocyclic ring. They are the sites for enzymatic cleavage and metabolism.
Alkene Moieties
Double bonds (C=C) are interspersed throughout the structure of Rapamycin. They foster its overall hydrophobicity and chemical reactivity.
Stereochemistry
The spatial organization of atoms in a molecule in three dimensions forms the Rapamycin structure. It is a molecule with multiple chiral centers. It gives start to different stereoisomers. However, naturally occurring Rapamycin exists predominantly as a single stereoisomer with a defined composition of chiral centers.
Major aspects of the stereochemistry of the Rapamycin structure are the next.
Chiral Centers
Rapamycin contains several chiral centers. They are carbon atoms bonded to four different substituents. These chiral centers are asymmetric. They can exist in two different compositions – R and S enantiomers.
The precise arrangement of substituents around each chiral center determines the overall stereochemistry and the structure of Rapamycin’s molecule.
Stereospecific Synthesis
The stereochemistry of Rapamycin structure is determined during its biosynthesis through a stereospecific enzymatic process.
Biosynthetic enzymes accelerate the assembling of the complex structure of Rapamycin in a highly controlled manner. This ensures the correct spatial arrangement of atoms and the formation of specific stereoisomers.
Conformational Flexibility
With the large and rigid macrocyclic structure of Rapamycin, this molecule exhibits some degree of conformational flexibility. This is provided by rotations around single bonds and flexibility in the macrocyclic ring.
This flexibility can influence the molecule’s interactions with biological targets and its medical properties.
Biological Activity
Biological activity and interactions with target proteins are defined by the stereochemistry of the Rapamycin structure.
Small changes in it can significantly impact the molecule’s properties. They can affect its power, selectivity, and mode of operation.
Reactions of the Organism
Active properties, absorption, distribution, and excretion are also influenced by the stereochemistry of the Rapamycin structure. Differences in stereochemistry can affect the molecule’s stability. Solubility and interactions with metabolic enzymes and transport proteins are also influenced.
Biological Targets and Mechanisms of Operation

Rapamycin molecule provides its chemical effects by interacting with a protein mTOR. By connecting to mTOR, this molecule oppresses its activity. By this action, it disrupts signaling pathways involved in cell growth, proliferation, and exchange processes.
The structure of Rapamycin and its mechanism underlies different therapeutic applications. It can be used in the healing of immunosuppression, cancer therapy, and the healing of certain rare diseases.
The biological activity, chemical properties, and possible medical applications are determined by the Rapamycin chemical structure.
By unraveling the structure of Rapamycin, researchers can gain significant information about the mechanisms of operation. They can explore new avenues for drug development and medical research. Investigations into the Rapamycin properties continue. However, the Rapamycin chemical structure remains a focal point for unlocking its full possibilities in molecules and beyond.






